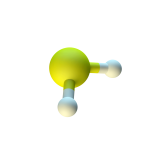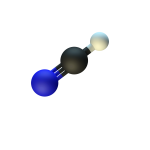The first land on Earth was probably volcanic, forming island arcs in a vast ocean. Springs, ponds, or lakes in volcanic regions were likely environments for jump-starting life.
The early atmosphere had no oxygen. It consisted mainly of nitrogen and carbon dioxide, with smaller amounts of hydrogen, water, and methane.
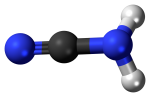
Lightning, asteroid impacts, and ultraviolet light from the Sun acted on the atmosphere to generate hydrogen cyanide; a compound of hydrogen, carbon, and nitrogen.

Meet the molecules
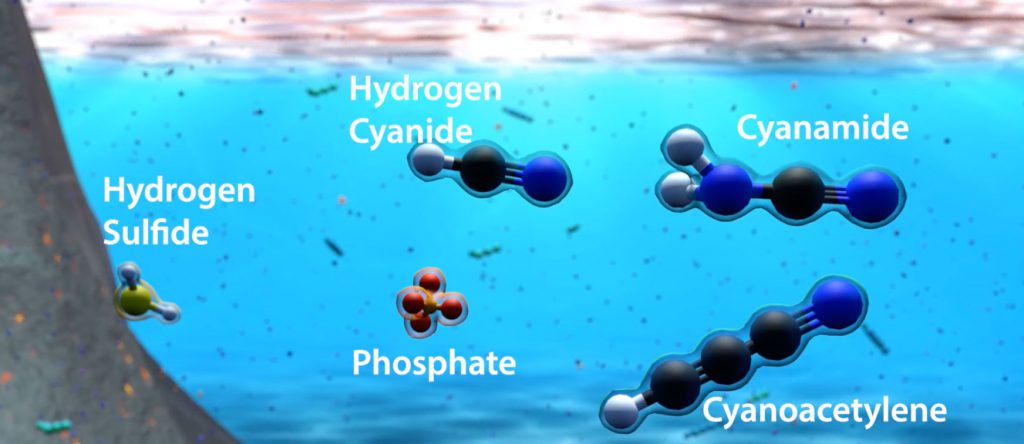
- Formed deep inside the Earth and released in great volcanic eruptions
- Smells like rotten eggs and is extremely toxic to humans
- Some bacteria produce hydrogen sulfide, while others live off it!
- Formed as meteorites streamed through the atmosphere of the young Earth – carbon- and nitrogen-containing molecules in the atmosphere were broken apart by the intense heat surrounding the meteorites and then recombined into hydrogen cyanide
- Also extremely toxic to humans, but tastes and smells like bitter almonds to some!
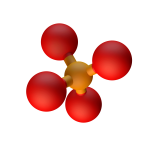
- Formed when phosphorus-containing minerals such as schreibersite were eroded and dissolved away by flowing water
- Phosphates are necessary to human, animal and plant life on Earth, so it fortunately doesn’t smell bad and isn’t toxic!
- When hydrogen cyanide came into contact with iron on the planet’s surface, it was able to react to produce a variety of different molecules, including hydrogen cyanamide
- Modestly toxic to humans, but used as a fertiliser in agriculture
- Formed by lightning discharges through the mixture of nitrogen and methane in the atmosphere of the early Earth
- Has been detected in interstellar clouds and in the atmosphere of Saturn’s moon Titan!
Read on to find out more about how early Earth chemistry went from molecules