The simple molecules found on the early Earth were capable of reacting together in different ways to produce the building blocks of life.
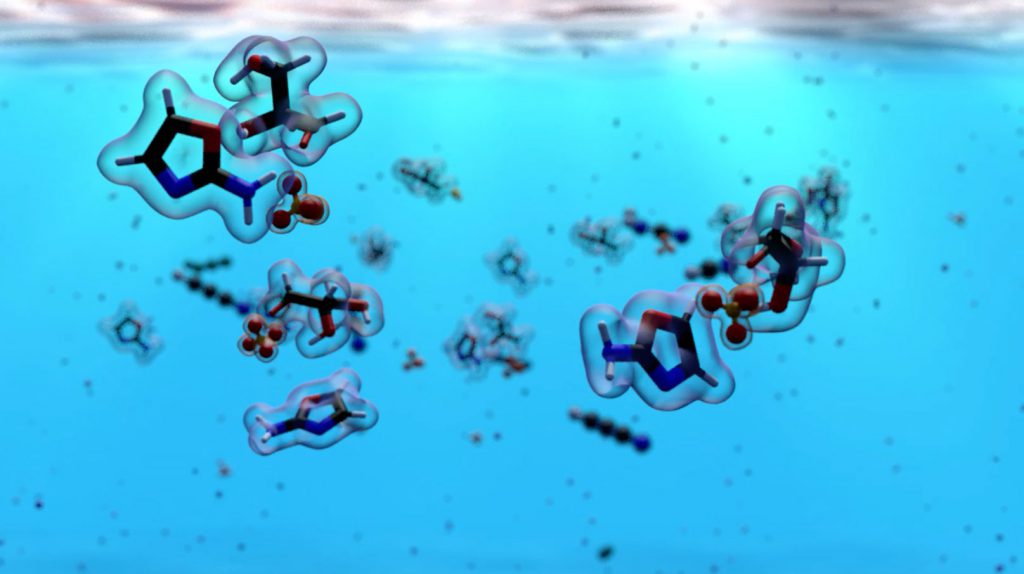
Three important building blocks are amino acids, lipids, and nucleotides. Each is used by our cells to make more complex molecules and structures necessary for life. But how each of these is made in modern biology is very different to how they were made at the emergence of life.
Meet the building blocks
- On the early Earth, amino acids could have formed from hydrogen cyanide, water, and ammonia, using sunlight as an energy source
- Amino acids are used to make proteins by reacting them together in a long chain
- The human body has about 100,000 different types of proteins, performing a huge number of different functions
- In our cells, amino acids can be acquired from protein in our diet or built from other nutrients
- On the early Earth, lipids could have been produced upon the exposure of mixtures of simple molecules, such as hydrogen cyanide, hydrogen sulfide, and phosphate to sunlight and heat
- Lipids combine into membrane bubbles that form the edge of our cells as well as separated compartments within our cells
- Many vitamins, including vitamin A and D, are fat-soluble, meaning that they must be associated with fat/lipid molecules in order to be absorbed by our body
- An intermediate molecule on the route to production of lipids is glycerol, which is widely used as a sweetener in the food industry and to make longer-lasting soap bubbles
- In our cells, a wide variety of proteins are needed to assemble water-insoluble lipids from simple, water-soluble precursors such as acetic acid and phosphate-containing molecules
- On the early Earth, nucleotides could have been formed from hydrogen cyanide, when exposed to UV radiation and combined with hydrogen sulfide and phosphate
- Nucleotides join together to form strands of RNA or DNA that carry and convey information that codes for the sequence of amino acids in proteins and defines features like our eye colour
- Your DNA could stretch from the Earth to the Sun and back about 600 times
- One simple molecule used on the path to making nucleotides is urea, which is widely used in fertilisers
- In our cells, nucleotides are formed through the chemical reaction of phosphate, a ‘pentose’ sugar (a sugar containing 5 carbon atoms, either ribose or deoxyribose), and a nitrogen-containing base, with proteins required to drive this process
What we work on
The Sutherland lab at the MRC Laboratory of Molecular Biology is focused on understanding which geochemical scenario could provide the right environment for the emergence of life.
We have found that the major building blocks of life (RNA and DNA nucleotides, amino acid and lipid precursors) could emerge in what we call the “cyanosulfidic scenario”.
In simple terms, hydrogen cyanide, together with hydrogen sulfide and phosphate, would provide the major building blocks of life when mixed in water and exposed to sunlight.
We are now exploring the chemical origins of genetically coded translation, i.e. the process by which the DNA or RNA code is converted into proteins, and the primordial lipids could assemble into functional membranes to surround the first cells, known as protocells.
Read on to find out more about how early Earth chemistry went from building blocks